Affinity Maturation or Degeneration for Custom Antibody
Catalog-Number:
BCHUAD
Description
Monoclonal antibodies have been approved to treat a variety of diseases. The binding characteristics of these therapeutic antibodies can be altered by maturation (gain of affinity) or degeneration (loss of affinity) techniques to confer different functionalities that will be useful to new areas of research and therapeutics. For example, a bispecific antibody would benefit from such an alteration to reduce its binding affinities to each single antigen, thereby acquiring an increased binding specificity to cells carrying both antigens
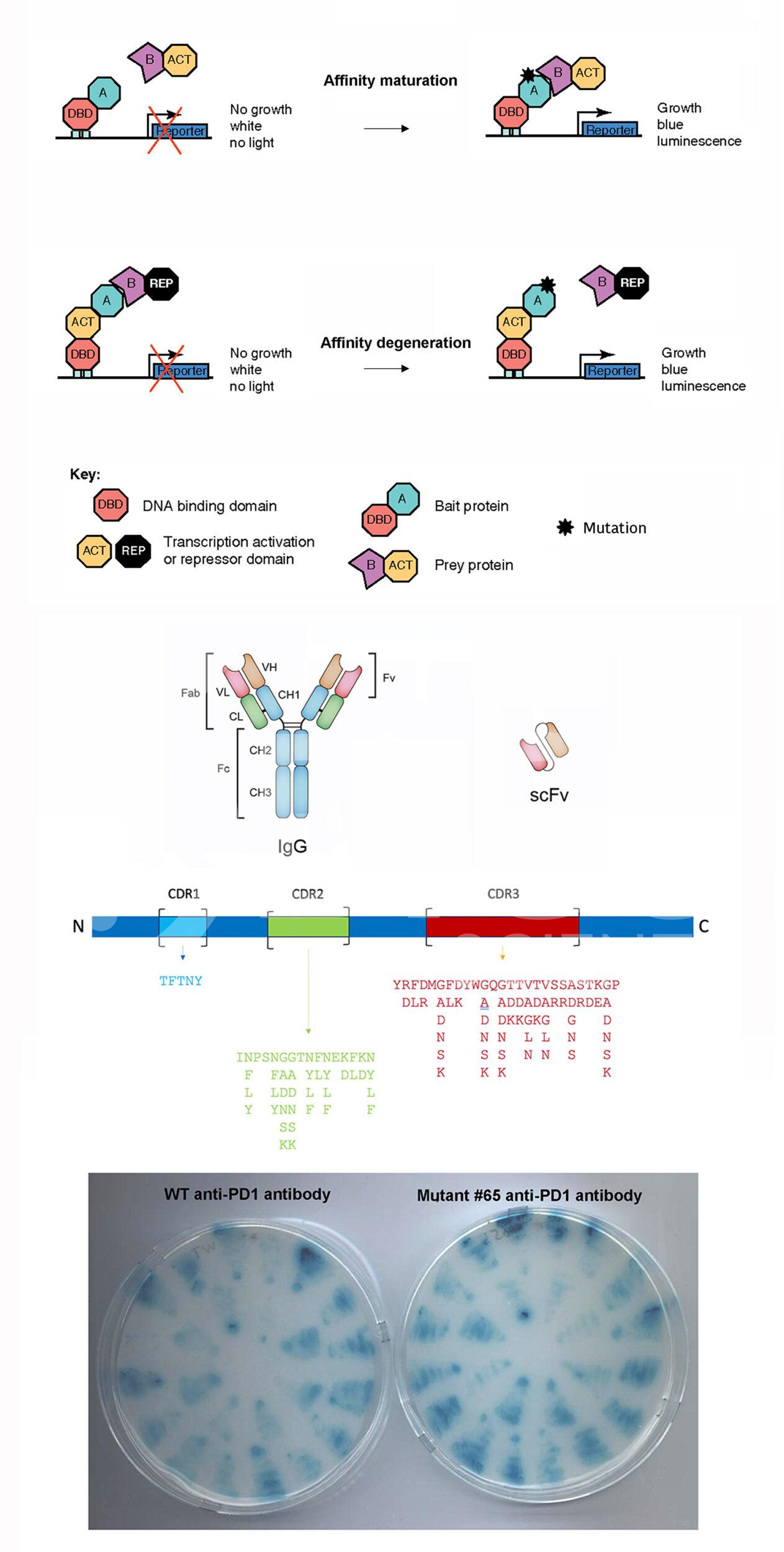
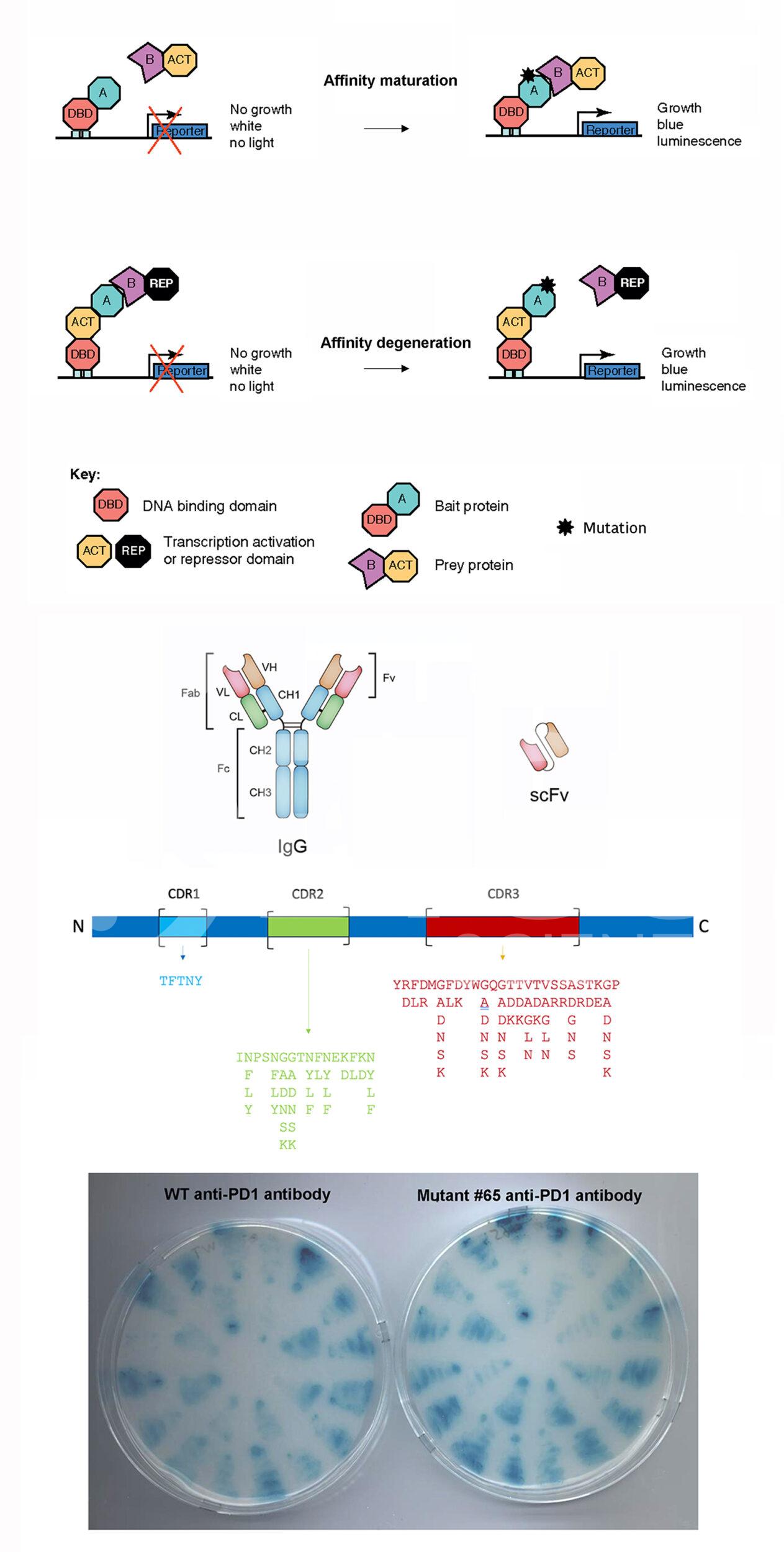
BiCell Scientific’s affinity maturation or degeneration service utilizes a unique and proprietary yeast 2-hybrid platform to screen mutations in the the complementarity-determining regions (CDRs) within the Fv domain of both heavy chain and light chain of the antibody molecule, in order to experimentally alter the binding affinities of custom-made or existing therapeutic monoclonal antibodies to suit a wide range of new applications.
Service Specification
- Molecular cloning of CDRs (CDR1, CDR2, and CDR3) from IgG heavy chain and light chain into yeast 2-hybrid library vector pY2H series, expressing the scFv form of fused Fv domains from heavy chain and light chain.
- Transform pY2H libraries into EBY100 YSD yeast strain.
- Perform yeast 2-hybrid screen with different growth stringency levels.
- Recover stringency tested yeast cells and plate them to form single colonies.
- Clone the scFv gene sequences from recovered yeast cells into lentiviral expression vector.
- Transfection of HEK293T cells with lentiviral expression vectors as well as vectors expressing gag–pol, rev and vsv–g.
- Harvesting and purification of lentivirus from cell culture medium.
- Transducing naive Sp2/0-Ag14 hybridoma cells with lentivirus encoding engineered IgG.
- Clonal selection and stable cell line establishment in 96-well plate.
- Validation of engineered IgG antibody binding characteristics in 96-well plate against antigen and selection of the desired clones with altered binding affinities.
- Affinity chromatography purification.
Contents
- Fully sequenced Fv domains of heavy chain and light chain of the engineered antibody
- One stable Sp2/0-Ag14 hybridoma cell line expressing engineered monoclonal antibody with the desired binding affinity
- 1 mg of affinity purified engineered monoclonal antibody
| Clonality: | Monoclonal |
|---|---|
| Isotype: | IgG |
| Purification: | Affinity Chromatography |
| Reactivity: | Human, Mouse, Rat |